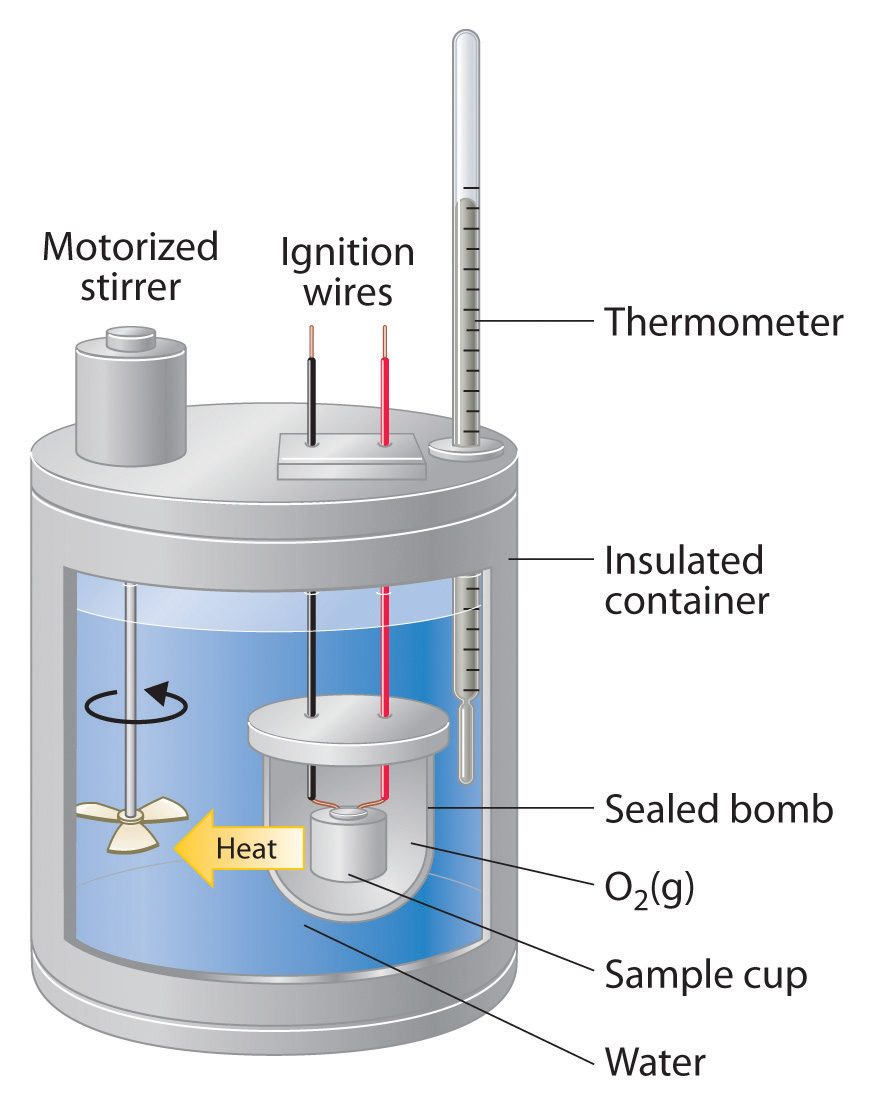
Calorimeter In Chemical Engineering . Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. The subset of differential calorimetry that deals with known heating or cooling rates is termed differential scanning. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. It uses devices called calorimeters, which measure the change in. For example, when an exothermic. Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for. This change can evoke change.
from 2012books.lardbucket.org
The subset of differential calorimetry that deals with known heating or cooling rates is termed differential scanning. It uses devices called calorimeters, which measure the change in. For example, when an exothermic. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for. This change can evoke change.
Calorimetry
Calorimeter In Chemical Engineering Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. It uses devices called calorimeters, which measure the change in. Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. The subset of differential calorimetry that deals with known heating or cooling rates is termed differential scanning. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. This change can evoke change. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system.
From 2012books.lardbucket.org
Calorimetry Calorimeter In Chemical Engineering This change can evoke change. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. For example, when an exothermic. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for. It. Calorimeter In Chemical Engineering.
From www.britannica.com
Calorimeter instrument Britannica Calorimeter In Chemical Engineering Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Reaction calorimetry is. Calorimeter In Chemical Engineering.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture Calorimeter In Chemical Engineering Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The subset of differential calorimetry that deals with known heating or cooling rates is termed differential scanning. It uses devices called calorimeters, which measure the change in. Reaction calorimetry is a powerful experimental method. Calorimeter In Chemical Engineering.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter In Chemical Engineering Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. For example, when an exothermic. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. A calorimeter is a device used to measure the amount. Calorimeter In Chemical Engineering.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter In Chemical Engineering Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. This change can evoke change. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. For example, when an exothermic.. Calorimeter In Chemical Engineering.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimeter In Chemical Engineering Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. For example, when an exothermic. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. The subset of differential calorimetry that deals with known heating or cooling rates is termed differential scanning. Calorimetric data from chemical reactions such. Calorimeter In Chemical Engineering.
From www.indiamart.com
Junkers Gas Calorimeter, For Lab, Automation Grade Automatic, ID Calorimeter In Chemical Engineering Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. A calorimeter is a device used to. Calorimeter In Chemical Engineering.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Calorimeter In Chemical Engineering Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. This change can evoke change. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. It uses devices. Calorimeter In Chemical Engineering.
From www.sealengineering.no
Differential scanning calorimeter Seal Engineering Calorimeter In Chemical Engineering This change can evoke change. The subset of differential calorimetry that deals with known heating or cooling rates is termed differential scanning. For example, when an exothermic. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. A calorimeter is a device used to measure the amount of heat involved. Calorimeter In Chemical Engineering.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimeter In Chemical Engineering Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. It uses devices called calorimeters, which measure the change in. This change can evoke change. The subset of differential calorimetry that deals with known heating or cooling rates is termed differential scanning. A calorimeter is a device used to measure. Calorimeter In Chemical Engineering.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimeter In Chemical Engineering For example, when an exothermic. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. It uses devices called calorimeters, which measure. Calorimeter In Chemical Engineering.
From www.chegg.com
Solved A bomb calorimeter, or constant volume calorimeter, Calorimeter In Chemical Engineering Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. For example, when an exothermic. It uses devices called calorimeters, which measure the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetric data from chemical reactions such. Calorimeter In Chemical Engineering.
From www.fauske.com
Reaction Calorimetry & Engineering Consulting Firm Fauske and Associates Calorimeter In Chemical Engineering Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. This change can evoke change. It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the. Calorimeter In Chemical Engineering.
From chemistrytalk.org
Calorimetry ChemTalk Calorimeter In Chemical Engineering A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. It uses devices called calorimeters, which measure the change in. Calorimeter is a device for the measurement of heat evolved or consumed at the. Calorimeter In Chemical Engineering.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Calorimeter In Chemical Engineering Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. Reaction calorimetry is a powerful experimental method widely used for reaction hazard assessment in chemical engineering for. This change can evoke change. It uses devices. Calorimeter In Chemical Engineering.
From www.scribd.com
calorimetry Chemical Engineering Chemical Process Engineering Calorimeter In Chemical Engineering For example, when an exothermic. This change can evoke change. It uses devices called calorimeters, which measure the change in. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. Reaction calorimetry strongly penetrated process development laboratories in the fine chemicals industry. The subset of differential calorimetry that deals with known heating or cooling. Calorimeter In Chemical Engineering.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter In Chemical Engineering The subset of differential calorimetry that deals with known heating or cooling rates is termed differential scanning. Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. It uses devices called calorimeters, which measure the change in. A calorimeter is a device used to measure the amount of heat involved. Calorimeter In Chemical Engineering.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimeter In Chemical Engineering Calorimeter is a device for the measurement of heat evolved or consumed at the change of state of the system. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetric data from chemical reactions such as reaction enthalpy, adiabatic temperature rise, and activation energy are. This change can evoke change. For example, when an exothermic.. Calorimeter In Chemical Engineering.